Introduction
Hypercholesterolemia is a condition which is presented by elevated levels of cholesterol. This in turn leads to the formation of plaques in the arteries and over time acts as a precursor for cardiovascular disorders (CVD). Cholesterol synthesis is regulated by a key enzyme HMG-CoA reductase through the Mevalonic acid pathway. Statins are known to inhibit this key rate limiting enzyme and in turn reduce cholesterol production.
NBI’s formulation composed of potent natural ingredients from 3 unique sources also have the same mechanism of action thus having a direct effect on cholesterol synthesis. Secondly, atherosclerosis and its effect on tissue oxidant stress is a precursor for CVDs. NBI’s anti-cholesterol formulation has also shown to reduce the reactive oxygen species and prevent the oxidation of lipids and cholesterol. Acting through these multiple mechanisms to prevent CVDs and with no proven side effects, NBI’s formulation rises as a clear alternative to statins. In silico studies were the primary step to shortlist active ingredients that can inhibit HMG-CoA reductase. The study established a significant binding energy which was higher in comparison to a standard statin drug of choice.
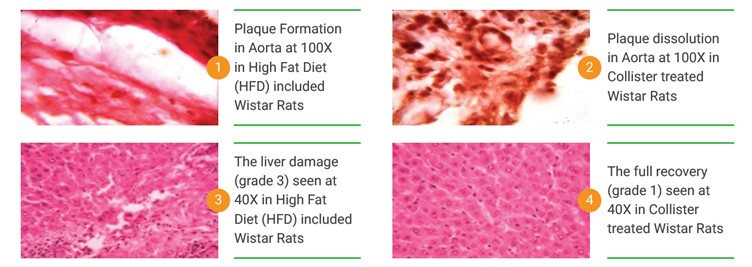
In vivo studies: Oral administration of NBI’s anti-cholesterol formulation in hypercholesterolemic rats is shown to reduce total cholesterol by a significant 22% over a period of 60 days. It also reduces total triglycerides by 26% over this period. The LDL-C levels are reduced by 17% as well. These results are more effective than the effect of statins by roughly two times delivering double the benefits. Positive results were observed when NBI’s formulation was given prophylactically and therapeutically. The direct positive impact of NBI’s anti-cholesterol formulation was evaluated through the examination of plaque reduction in high fat diet fed hypercholesterolemic rats. Histopathological evaluation of plaques and liver cells clearly showed reduction of plaques and recovery of fatty liver cells. The dual mechanism of NBI’s formulation was further complimented by its safety studies. The studies showed NO mortality of any of the animals and the histopathological examinations of all organs showed no damage at the cellular level.
Clinical studies: Phase I study of NBI’s anti-cholesterol formulation was carried in healthy human subjects to evaluate its bioavailability and establish safety. At doses as high as 800mg per day, the subjects were found to have no side effects and the formulation’s safety was established. Haematology, biochemical examination and lipid profile of blood samples collected from all subjects was well within limits thus extending the safety of the formulation.