Science
SLIDD, a novel and exceptional technology is composed of three essential ingredients that work independently to ensure the bioavailability of the active drug to the site of action, the COLON. Any drug enveloped with the SLIDD technology is dosed in small quantities and due to the mechanism of action of the components is delivered almost 100% to the colon. This has been proven through multiple studies – both in vitro and clinical. The strategic innovation team of NBI consists of Dr Saurabh Srivastava, Dr Aashish Gambhir and Dr Richa Srivastava.
The first excipient is selected based on their physical compatibility with the drug of interest such as compression, friability and stability, thus ensuring that the drug is packed well and does not disintegrate enroute to the colon. The second coating is an inert ingredient that is able to withstand the different pH and temperature conditions of the entire gastrointestinal tract (GIT). To enable transit support for faster movement (Such as Eudragit S100) across the human GIT alongwith managing the shape and hardness of the formulation that ensures this safe passage, the third ingredient is selected. In addition to the combinatorial effect of the 3 ingredients, the most unique feature of SLIDD is its capability to bring about a “drug shower” or “slow release of the drug” to enable systemic absorption of the drug in the colon for 24 to 48 hours.
The use of SLIDD technology may be expanded in combination with biosimilars, monoclonal, antibody and hormones, all of which would otherwise be sensitive to the conditions prevailing in the GIT. Its potential to by-pass metabolism by the liver helps reduce 50% of the side-effects. SLIDD technology uses natural ingredients that are approved for human use thus increasing its acceptability across regulatory bodies.
SLIDD Technology – In vitro
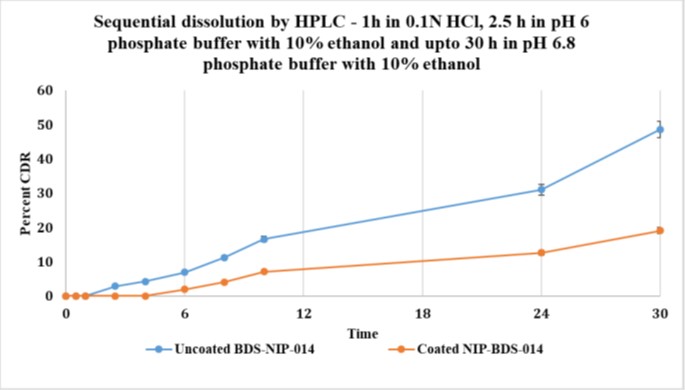
The efficacy of SLIDD technology was proven by in vitro as well as clinical trials, specifically targeting patients with inflammatory bowel disorders such as ulcerative colitis. It becomes a requirement to ensure that drugs that are given are able to reach the proximal end of large intestine. Most drugs are not able to meet this pre-requisite and only 5-20% bioavailability has been observed. Budesonide, a BCS class II, steroidal drug which acts as an anti-inflammatory agent suffers from poor aqueous solubility with only 40% absorption reported. Higher doses lead to adverse side effects such as headache, nausea, decreased blood cortisol, upper abdominal pain, fatigue, flatulence, abdominal distension, acne, urinary tract infection, arthralgia and constipation.
In vitro studies carried out at NIPER, a leading pharmaceutical institution in the country, provided data to establish the slow release of Budesonide when in formulation with SLIDD. Experiments were conducted by creating physiological conditions mimicking the colon and analysing the levels of active drug retention in each stage. The results indicated the effective controlled release of not more than 2% budesonide before reaching the Colon when Budenoside was coated with SLIDD ingredients. The formulation showed 0% release of budesonide in Phase-0 and Phase-I stomach and small intestine mimicked conditions. To establish the dissolution or disruption of Budenoside from the formulation, rat cecal (4%) was used to recreate rumen conditions. The disruption of tablet matrix was physically observed after completion of dissolution tenure when tablets were prepared using SLIDD technology. Analytical data also supported this observation of complete dissolution.

Read more about SLIDD >> Commercial >> Science >> Scintigraphy