×
Dr. Aashish Gambhir
Dr Aashish Gambhir is a dynamic physician obtaining his graduate degree in medicine from the prestigious MAMC medical College in Delhi. He specialised in super speciality subjects pertaining to modern medicine with significant clinical exposure. Later he obtained Diploma in Radiation Medicine (DRM), a post graduate qualification in the rapidly advancing field of nuclear medicine from the defence research institution (DRDO– INMAS). During this period, he obtained accolades, such as the gold medal for his paper on parathyroid Imaging. He developed this further at leading national institutions such as the Apollo hospitals, Chennai, where he received extensive clinical training in gamma camera/SPECT Imaging, PET imaging and nuclear Theragnostic while working with multiple radio tracers for and Investigative and therapeutic purposes. Dr Gambhir is certified by the Atomic Energy Review Board. (AERB). Over the last 12 years he has been operating as consultant and at various facilities in the country Including House of Diagnostics, Yashoda Hospitals, Narula Diagnostic and Ganesh Diagnostics which offer a full suite of nuclear medicines studies for patients.
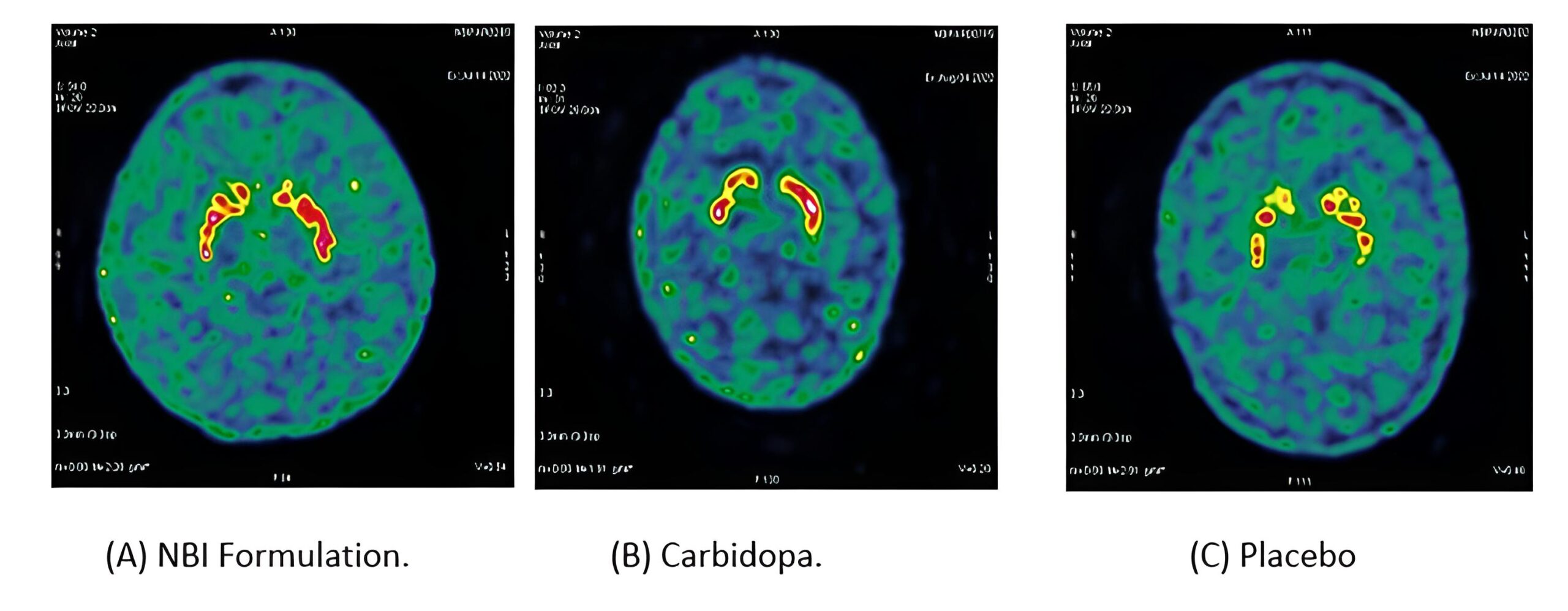
He has been actively involved in pharmaco-scintigraphy work and remains an active member of the society for nuclear medicine in India. He has an active understanding of plant based long chain molecules, which often promise a reduction of side-effects (as opposed to chemically synthesised drugs). At NBI Biosciences he has led and been closely associated with experiments using Scintigraphy Showing the efficacy of certain plant derived molecules replacing carbidopa with greater efficacy in Parkinson’s disease. He has also Investigated the delivery technology (SLIDD) to the human colon. Both of these have been patented by NBI Biosciences.